-
Products and Solutions

Efficient and reliable products for all your unique requirements.
-
Support & Services
We offer services that prioritize customer satisfaction at every step.
- Partners
- Industries
-
About Us
Ask us on Social Media Use our social channels to search for answers and ask questions
- Blogs


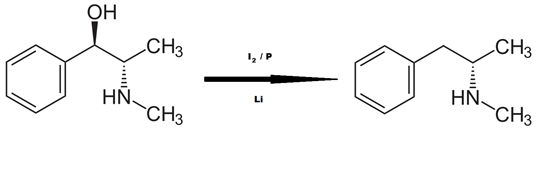
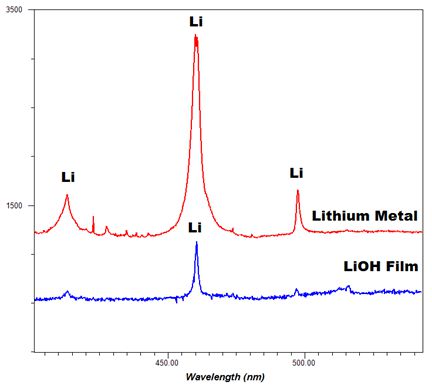
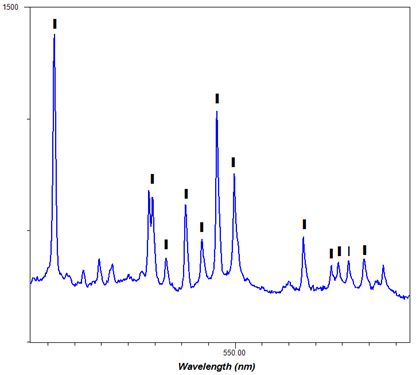
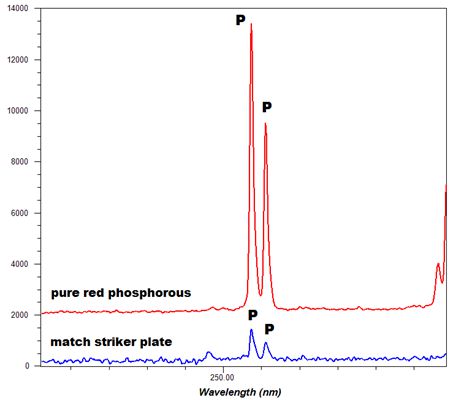
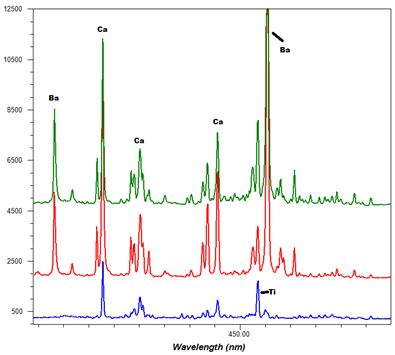
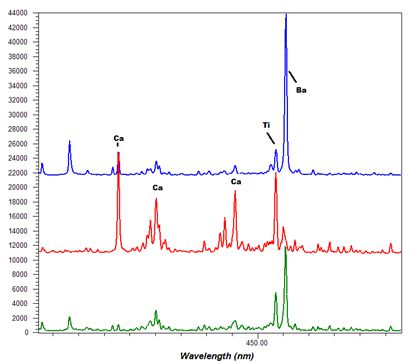
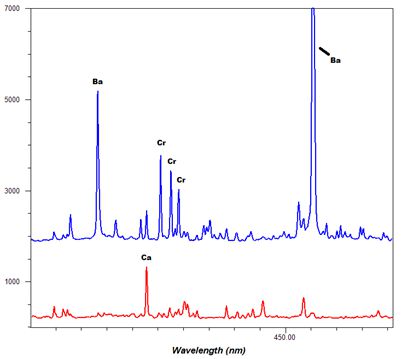
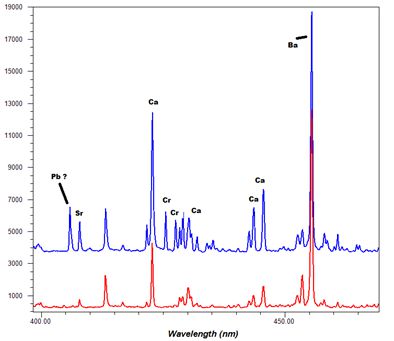


-Thumbnail.png)